Multiple myeloma (MM) is a genetically heterogeneous disease, lacking molecular markers for patient stratification and prediction of clinical response. We have created a unique platform for clinical-informed decisions, research advancement, and preclinical compound testing called the “Ex Vivo Mathematical Myeloma Advisor” (EMMA). In this pipeline, MM patients who undergo bone marrow (BM) biopsy and consent to participate in the Total Cancer Care protocol have their clinical data (demographics, therapies received, lab results, clinical history, etc.) abstracted, along with the molecular characterization (FISH/cytogenetics, RNA-Seq, and WES) of their disease.
In parallel, fresh BM-derived MM blasts are combined with stromal cells in a collagen-based matrix containing patient plasma and used in an ex vivo drug screening assay. The cells are seeded into 384 or 1536-well plates and exposed to serial dilutions of up to 127 single agents and combinations. The entire setup is imaged in bright field every 30 minutes for a duration of 6 days. Image analysis is used to convert membrane integrity into survival curves, and AUC and LD50 values are estimated. The pattern of these curves facilitates the identification of resistant subclones. This data is further combined with augmented synergy models that are parameterized with PK/PD data for each drug. This integration permits the simulation of “in silico clinical trials”, which are compiled into reports that assist clinicians in selecting the best treatment for their patients. In addition, the immune population of the BM aspirate is characterized by flow cytometry, and soluble factors in the patient's BM plasma are quantified by ELISA.
The integration of clinical, molecular, and phenotypic data with image analysis and machine learning tools presents a unique opportunity for finding molecular signatures of drug response (Fig. 1). For instance, our pipeline can be utilized to identify clinical factors (such as ethnicity, age, etc.) and molecular features (mutations, cytogenetic abnormalities, enriched transcription factors, differentially expressed biological pathways, etc.) associated with drug resistance/sensitivity. Additionally, it enables the determination of the mechanisms of action of new compounds and the prediction of potential synergistic/antagonistic drug combinations. The longitudinal acquisition of data in our non-destructive ex vivo drug screen assay facilitates the exploration of drug interaction dynamics over time, enabling the pinpointing of the moment when synergism occurs at maximum intensity. This, in turn, allows us to propose optimized temporal-based therapeutic regimens for clinical use. Up to this point, our pipeline has molecularly characterized nearly 1,000 MM samples, and EMMA has been applied to over 600 samples, forming one of the world's most extensive and comprehensively characterized MM cohorts.
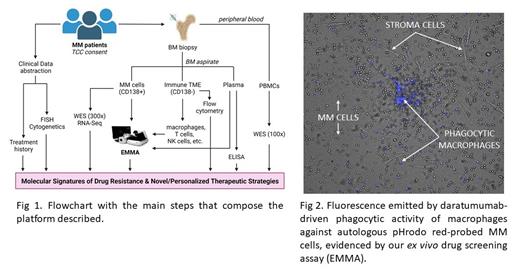
While EMMA has proven effective in quantifying the activity of monoclonal antibodies, ongoing efforts are focusing on incorporating individual immune cell components to assess ex vivo activity in the context of evolving cellular immunotherapies. As a proof of principle, we demonstrate the system's capability to measure the effect of daratumumab (CD38+ antibody) on autologous macrophage-mediated MM cell death, thereby distinguishing it from apoptosis caused by direct antibody binding (Fig. 2). Additionally, we evaluate the influence of teclistamab (T-cell engager CD3/BCMA bispecific antibody) on CD8+ T cell activity against autologous MM cells, as well as the efficacy of CAR-T cells in killing MM cells. Through the testing of standard-of-care drugs alongside immune agents, our pipeline has identified the combination of bortezomib + daratumumab as a synergistic approach with significant translational potential, warranting further investigation.
In summary, our state-of-the-art platform facilitate drug discovery and unravel the intricate biological phenomena underlying drug interactions in MM. The advantages offered by our system are threefold: i) it enables pharmaceutical companies to test new compounds in a bona fide, patient-derived model; ii) it assists clinicians in selecting the best therapy for their patients, and iii) it helps prevent cancer patients from receiving non-suitable therapies. This approach can also be promptly applied to other liquid cancers, such as leukemia and lymphoma.
Disclosures
Hampton:Aster Insights: Current Employment. Shain:BMS: Consultancy, Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm: Honoraria, Research Funding; Sanofi Genzyme: Honoraria, Speakers Bureau; Adaptive: Consultancy, Speakers Bureau; Abbvie: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal